Audit Services
We offer specialized factory audit services tailored to ensure compliance with WHO GMP and government standards.
Explore MoreQMS & Risk Management
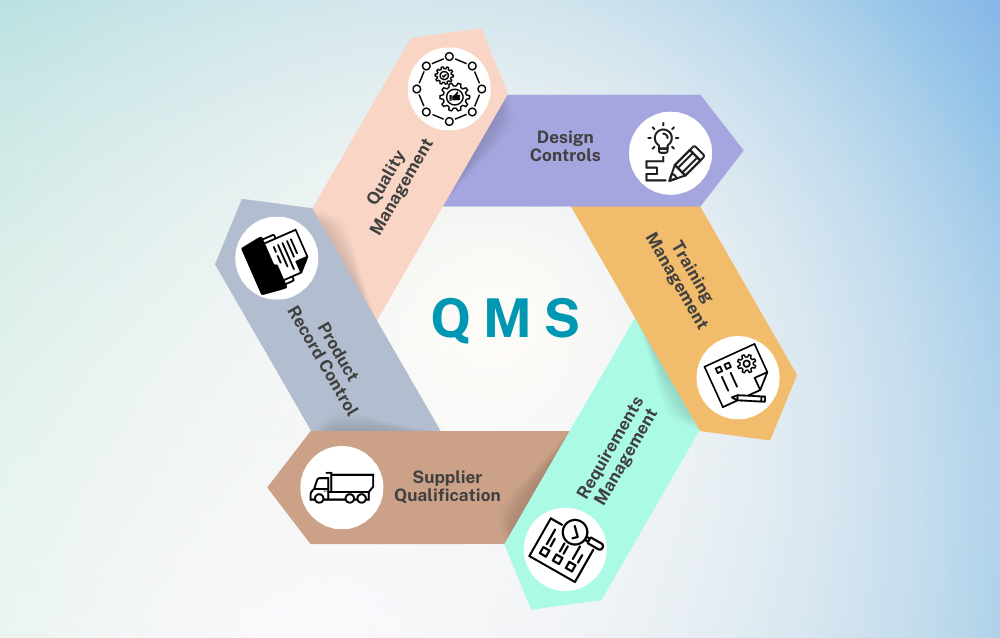
We help medicine manufacturing firms implement Quality Management System (QMS) as per WHO guidelines.
Explore MoreDocumentation Services
We offer support in managing and updating essential documentation needed for medical industry regulatory compliance.
Explore MoreMachine Selection
We understand that the right machinery is pivotal for optimal pharmaceutical production.
Explore MoreTest License on Form 29
This license is granted by State FDA for manufacturing drugs for purposes of examination.
Explore MoreLoan License for Drugs Manufacturing
A Loan License allows you to manufacture drugs at a third party’s established facility. It’s essential for businesses looking to expand without heavy investment in new factories.
Explore MoreDrugs Manufacturing License
Pharmadocx Consultants provides regulatory work for the grant of all types of Drugs Manufacturing Licenses in India – Form 25
Explore MoreFactory Layout Design Service
We visit your premises, take measurements, and design an industry-standard layout.
Explore MoreFactory Site Selection
Choose the right place for your pharma factory with our help. We know what works in India.
Explore MoreWe help medicine manufacturing firms implement Quality Management System (QMS) as per WHO guidelines. Quality Management Systems (QMS) and Risk Management are critical components in pharmaceutical manufacturing, ensuring product consistency, safety, and compliance with international standards. Pharmadocx Consultants excels in establishing robust QMS and Risk Management protocols as per WHO directives. We help structure your operations to meet global benchmarks, implementing comprehensive strategies that encompass everything from raw material